Abstract
Background: Therapy-related myelodysplastic syndrome (t-MDS), a long-term complication of cancer survivors who received cytotoxic therapy, is characterized by high-risk genetics and poor outcome. Allogeneic hematopoietic cell transplantation (alloHCT) represents the only potentially curative modality for t-MDS, but the prognostic impact of pre-transplant genetics and clinical features has not been fully characterized.
Methods: In this retrospective IRB-approved study, a total of 266 consecutive patients with diagnosis of t-MDS (n=67) or de novo MDS (N=199) who received allogeneic HCT from matched sibling (n=111) or unrelated donors (n=155) at the City of Hope between January 2000 and October 2014 were analyzed. t-MDS was defined solely based on past history of exposure to any cytotoxic chemotherapy and/or radiotherapy administered for prior malignant or non-malignant conditions. Patient's DNA was extracted from whole blood samples before HCT. All patients had ≥ 20% circulating myeloid lineage cells at the time of the sample collection. A next generation sequencing (NGS)-based 72 gene panel was used to detect somatic mutations and cytogenomic microarray (CMA) was performed using the Affymetrix CytoScan HD platform.
Results: As compared to de novo MDS cohort, t-MDS cases were less frequently categorized by WHO classifications as RAEB-1/2 (39.1% vs 47.0%; p=0.004) and had higher risk cytogenetics (p=0.00003), lower blasts% at the time of HCT (p=0.03), higher IPSS score at diagnosis (p=0.03) and more frequently received reduced intensity conditioning (mostly fludarabine+melphalan) HCT (94.0% vs 70.4%; p=0.00003).
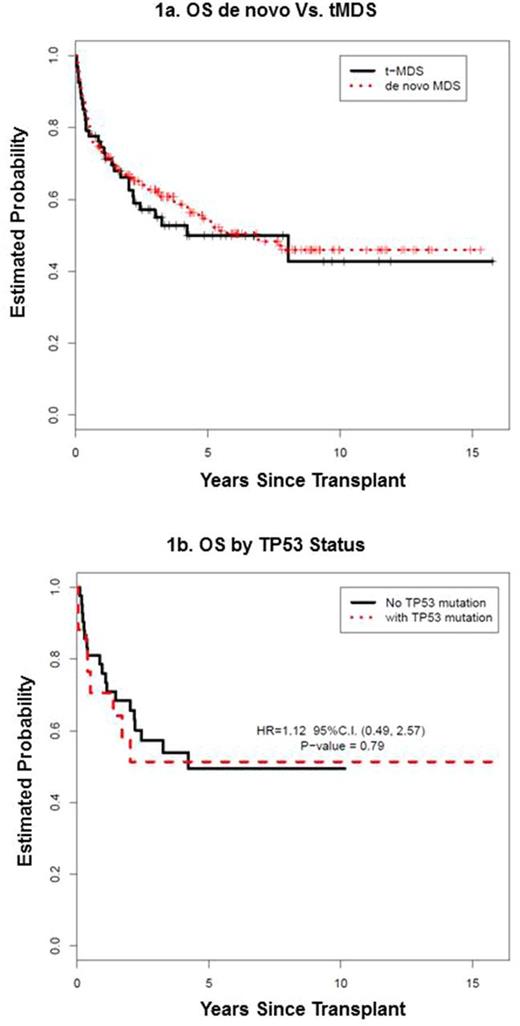
After a median follow-up of 4.8 years (range, 0.5-15.8) for surviving patients, the 5-year overall survival (OS) for the entire cohort was 52.8% (95% CI: 46.2-59.4%). There were no differences in the 5-year OS (49.9% vs. 53.9%, p=0.61), relapse-free survival (RFS) (47.2% vs. 49.5%, p=0.68), non-relapse mortality (NRM) (30.2% vs. 26.8%, p=0.48) and relapse (22.6% vs. 23.7%, p=0.81) rates between t-MDS and de novo patients (Figure 1a). In a multivariate model, prior cytotoxic therapy before t-MDS diagnosis did not impact survival (p=0.7) in the whole cohort. For t-MDS (n=67),older age was associated with trend toward lower OS (HR: 1.04 for each year, p=0.06) and lower RFS (HR: 1.04, p=0.05). More recent HCT era was associated with superior OS (HR: 0.27, p=0.02 for 2005-2009 and HR: 0.21, p=0.002 for 2010-2014). Karyotypes, IPSS score and percentage of marrow blasts before alloHCT were not independently associated with OS or RFS.
Of the 67 t-MDS patients, 60 had available pre-HCT DNA samples; 43 (72%) of them had at least one detectable gene mutation. Among patients with detectable mutations, the median number of mutations per case was 2 (range: 1-6). The most common mutated gene was TP53, which was present in 18 (30%) cases. Of 18 cases with somatic mutations involving TP53 gene, 5 (28%) had multiple distinct TP53 mutations (two mutations: n=4, three mutations: n=1). Other more common mutations (observed in ≥ 4 cases) were RUNX1 (12%), TET2 (8%), U2AF1 (8%), ASXL1 (8%), DNMT3A (7%) and SETBP1 (7%). Additional non- TP53 mutations (U2AF1, RUNX1, ASXL1, TET2, and STAG2) were observed in 33% TP53 -mutated cases. TP53 -mutations were more frequently associated with complex and/or monosomal karyotype compared to TP53 -wild cases (38% vs. 78%, p=0.02).
Contrary to previous reports, presence of mutations in TP53 or mutations in any other of the high-risk genes (EZH2, ETV6, RUNX1, ASXL1 : n=29, 48%) did not significantly impact either OS (Figure 1b) or RFS. In 3 cases of MDS, TP53 alterations were found only by CMA but not by NGS. Re-classifying these additional del17p cases onto the TP53 mutated group still showed similar OS between TP53 mutated and TP53 unmutated groups
Conclusion: AlloHCT is a curable treatment for patients with t-MDS with similar long-term survival to de novo MDS despite higher-risk features. While TP53 alteration was the most common mutation in t-MDS, the finding was not detrimental in our case-series.
Khaled: City of Hope: Research Funding; Daiichi Sankyo, Inc: Other: Travel Support. Salhotra: Kadmon: Consultancy. Stein: Stemline: Consultancy; Amgen: Consultancy, Speakers Bureau. Pillai: Trillium Therapeutics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal